Zinc and copper give nanocapsules blue fluorescence
17 July 2012
Tokyo Institute of Technology (Tokyo Tech) researchers have developed blue-fluorescent molecular nanocapsules by simply mixing metal ions and bent organic blocks. The nanocapsules have potential applications as sensors, displays, and drug delivery systems.
Michito Yoshizawa, Zhiou Li, and collaborators at Tokyo Tech synthesized molecular capsules of about 1 nanometer size with an isolated cavity using inexpensive and environmentally safe zinc and copper ions. In sharp contrast to previous molecular capsules and cages composed of precious metal ions such as palladium and platinum that show only poor fluorescence, these nanocapsules emit blue fluorescence with 80% efficiency.
The researchers expect to be able to prepare multicolour fluorescence composites by the simple insertion of appropriate fluorescent molecules into the isolated cavity of the nanocapsules.
Fluorescent capsules
Fluorescence has widespread applications, helping researchers to understand issues in the fundamental sciences and develop practical materials and devices. Among the useful fluorescent compounds in development, capsule-shaped molecular architectures, which possess both strong fluorescent properties and a nanometer-sized cavity, are particularly promising.
Molecular cages and capsules can be prepared through a simple synthetic process called co-ordinative self-assembly. However, most of them are composed of precious metal ions such as palladium and platinum, and are non-emissive due to quenching by the heavy metals.
Now, Michito Yoshizawa, Zhiou Li, and co-workers from the Chemical Resources Laboratory at Tokyo Institute of Technology report novel molecular nanocapsules with the M2L4 composition (where M represents zinc, copper, platinum, palladium, nickel, cobalt, and manganese). Their zinc and copper capsules, in particular, display unique fluorescent properties.
The M2L4 capsules self-assemble from two metal ions and four bent ligands that include anthracene fluorophores (fluorescent parts). X-ray crystallographic analysis verified the closed shell structures where the large interior cavities of the capsules, around one nanometer in diameter, are shielded by eight anthracene panels.
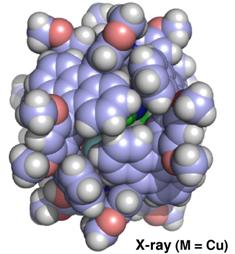
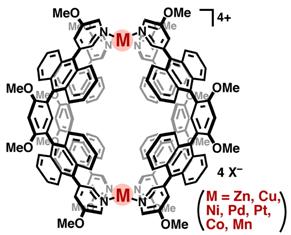
The structure of a molecular nanocapsule
The zinc capsule emitted strong blue fluorescence with a high quantum yield (80%), in sharp contrast to the weakly emissive nickel and manganese capsules and the non-emissive palladium, platinum, and cobalt capsules. The fluorescence of the copper capsule, on the other hand, depends on the solvent; for example, it shows blue emission in dimethyl sulfoxide but no emission in acetonitrile.
This study is the first to show such emissive properties of molecular capsules bearing an isolated large cavity. The researchers believe their nanocapsules could have novel applications in devices such as chemosensors, biological probes, and light-emitting diodes.
Reference
Zhiou Li, Norifumi Kishi, Kenji Yoza, Munetaka Akita, Michito Yoshizawa, “Isostructural M2L4 Molecular Capsules with Anthracene Shells: Synthesis, Crystal Structures, and Fluorescent Properties”, Chemistry, 18, 8358 (2012). DOI: 10.1002/chem.201200155.