Endomagnetics wins CE Approval for Sienna+ Tracer for breast cancer care
19 January 2012
Cambridge, UK. Endomagnetics has gained EU approval for the sale of its magnetic tracer material, branded Sienna+ with the award of the CE Mark. The injectable tracer is used in combination with the ultra-sensitive SentiMag instrument in sentinel lymph node biopsy (SLNB), currently the standard of care in tracking the spread of breast cancer.
The SentiMag instrument itself has also been CE approved, so clinicians across Europe now have available a complete system to be used in place of conventional radioisotopes. The need for special handling of radioactive materials means that many hospitals and clinics are not able to use them. As a result, as many as half of the patients in the West who would benefit from the SLNB technique are not able to access it, and availability is much worse still in other parts of the world.
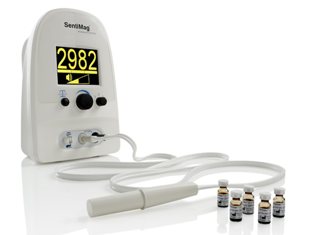
The SentiMag system
Endomagnetics’ development of the SentiMag and Sienna+ system allows best practice SLNB to be performed anywhere, by any practitioner, without substantially changing their working practice. The combined system offers reduced workflow complexity with a corresponding reduction in procedural cost.
“Achievement of CE approval for Sienna+ is an important milestone for Endomagnetics and our ability to deliver a complete solution to the market,” says Dr Eric Mayes, CEO of Endomagnetics. “It demonstrates that the system has met rigorous EU safety, health and environmental requirements.”
Source: Endomagnetics