St Jude gains CE mark for implanted neurostimulation device for migraine
12 September 2011
St. Jude Medical, Inc. (NYSE:STJ), has received the industry’s first regulatory approval for the use of an implanted neurostimulation device for patients with intractable chronic migraine.
The company received European CE Mark approval for its Genesis neurostimulation system for peripheral nerve stimulation (PNS) of the occipital nerves for the management of the pain and disability associated with intractable chronic migraine.
This type of migraine is defined as headache lasting at least four hours per day for 15 or more days per month, causing at least moderate disability, and not responding to three or more preventive drugs.
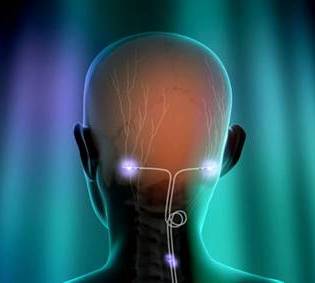
PNS therapy for this condition involves the delivery of mild electrical pulses to the occipital nerves that are located just beneath the skin at the back of the head. A small electrical lead or leads are placed under the skin and connected to the neurostimulator which produces the pulses of stimulation.
“As a professor and practicing neurologist who works with these patients on a daily basis, I see firsthand the challenges they face in trying to manage their pain and disability and how chronic migraine impacts their lives and their families,” said Dr. Stephen D. Silberstein, past president, American Headache Society, director of the Jefferson Headache Center, and the principal investigator in a recent St. Jude Medical chronic migraine clinical trial. “Through my participation in this study, I have observed the life-changing potential this therapy offers chronic migraine patients.”
The CE Mark approval was supported by the results of St. Jude Medical’s chronic migraine study, a randomized, double-blind, controlled study that collected data from 157 patients. On average, participants enrolled in the study suffered from headache 26 days per month.
The largest clinical study to date evaluating PNS to treat chronic migraine utilized various measures including the Migraine Disability Assessment (MIDAS) questionnaire, subjective assessment scales and daily patient diaries to report headache intensity, frequency, duration and medication use.
At 12 weeks, patients in the active group reported an average of seven fewer headache days a month as measured by the MIDAS questionnaire compared to only a one day per month decrease in the control group (non-stimulation group). In addition, overall disability as measured by MIDAS demonstrated that participants in the active group showed a 41% improvement after 12 weeks of stimulation, compared to a 13% improvement in the control group.
Results at one year included:
- 65% of patients reported excellent or good pain relief;
- 88% said they would recommend the procedure to someone else;
- 68% of patients expressed that their quality of life had improved;
- 67% were satisfied or very satisfied with the results of their procedure.
Results of the major study endpoints were presented in abstract and poster format at the International Headache Congress in Berlin in June 2011. Study data will be submitted for publication in medical journals later this year and early 2012.
“This CE Mark is the first approval by a regulatory body for the use of neurostimulation to manage the debilitating symptoms of intractable chronic migraine and provides a new option for patients who have generally exhausted all other treatment options,” said Chris Chavez, president of the St. Jude Medical Neuromodulation Division. “For more than six years we have worked with our investigators to develop and evaluate this life-changing therapy. We will continue to work with regulatory authorities to secure approvals in order to offer this therapy option to patients throughout the world.”
Source: St Jude Medical