New low-energy cardiac defibrillation method developed
15 July 2011
An international team of scientists has developed a new low-energy method for terminating life-threatening cardiac fibrillation of the heart.
They have shown that their new technique called LEAP (low-energy anti-fibrillation pacing) reduces the energy required for defibrillation by more than 80% compared to the current conventional method. Their discovery opens the path for the painless therapy of life threatening cardiac fibrillation.
The researchers are from the Max-Planck-Institute for Dynamics and Self-Organization (Göttingen, Germany), Cornell University (Ithaca, New York) the Ecole Normale Supérieure de Lyon (France), the University Medicine Göttingen (Germany), the Rochester Institute of Technology (USA), and the Institut Non-Linéaire de Nice (France). The results are describe their results in the current issue of Nature [1].
In a healthy heart, electrical pulses that propagate across the heart muscle in an orderly fashion control the organ’s movements: at regular intervals the heart’s ventricles and atria contract and relax again. In the case of cardiac arrhythmia, however, this does not work reliably. Here, electrical pulses may propagate throughout the heart chaotically, disabling the regular heartbeat and thus preventing the body from being properly supplied with blood. The most common cardiac arrhythmia is atrial fibrillation, which affects more than 10 million people in Europe and US.
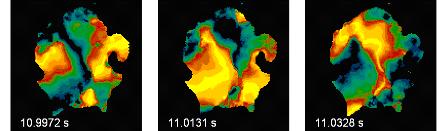
Spatial-temporal excitation pattern during
cardiac fibrillation on the surface of heart (field of view 6 x 6
cm2). Colour code: black = resting, yellow = excited. Credit: MPI
for Dynamics and Self-Organization
For patients suffering from chronic atrial fibrillation there is one reliable solution: a defibrillation. A strong electric pulse, which patients perceive as painful and which can damage the surrounding tissue forces the heart back into its regular beating. The international team of scientists led by Stefan Luther from the Max Planck Institute and Flavio Fenton from Cornell University has proposed a new method. Using a cardiac catheter the researchers create a sequence of five weak electrical signals in the heart. “Only a few seconds later, the heart beats regularly again”, says Luther describing the team’s newest results.
Even though LEAP and standard defibrillation seem to work similarly at first sight, they initiate completely different processes within the heart. “The classic defibrillator works by using a very strong electric field that excites all cells of the organ. In contrast, LEAP uses low-energy pulses to synchronize the tissue”, says Fenton. For a short moment they can no longer transmit any electrical signals; the chaotic activity is terminated. “Afterwards, the heart resumes its normal, regular beating. The situation can be compared to turning a malfunctioning computer off and on again,” says Robert Gilmour from Cornell University.
The new method terminates the turbulent electric activity within the heart step by step. “Our most important allies are natural heterogeneities within the heart such as blood vessels, fatty tissue or fibrotic tissue”, says Eberhard Bodenschatz from the Max Planck Institute. In experiments and computer simulations the researchers were able to show that these heterogeneities can act as the origins for synchronizing waves. “Quite weak electrical pulses suffice to stimulate the cells in these regions”, says Alain Pumir from Lyon. With every additional pulse more heterogeneities are activated thus gradually suppressing chaotic activity. “The heterogeneities act as small control sites that – once activated – can “reprogram” the entire organ”, adds Valentin Krinsky from Nice.
In principle, the results also apply for defibrillation of ventricular fibrillation, a life-threatening arrhythmia, which is terminated only by external and implantable defibrillators. For a large number of patients wearing implantable cardioverter-defibrillators (ICD) the new technique may eliminate pain, improve the success rate of treatment, prolong battery life and therefore reduce the need for surgical device exchanges.
"The development of LEAP is a groundbreaking result and an outstanding example of successful interdisciplinary collaboration between physicists and physician-scientists, with immediate impact on the development of novel therapies for life-threatening cardiac arrhythmias", says Markus Zabel from the University Center Göttingen. The ideas leading to LEAP were first developed by asking elementary physical questions about the interaction between electric field and cardiac tissue; the results of earlier theoretical work in physics, in particular in the French National Center for Scientific Research (CNRS), may be finding their way to clinics. Indeed, “we are working to get this to the patient as fast as possible”, adds Gerd Hasenfuss, the head of the Heart Center Göttingen.
Reference
Stefan Luther, Flavio H. Fenton, Bruce G. Kornreich, Amgad Squires, Philip Bittihn, Daniel Hornung, Markus Zabel, James Flanders, Andrea Gladuli, Luis Campoy, Elizabeth M. Cherry, Gisa Luther, Gerd Hasenfuss, Valentin I. Krinsky, Alain Pumir, Robert F. Gilmour Jr., Eberhard Bodenschatz. Low-energy Control of Electrical Turbulence in the Heart. Nature, 14 July 2011.
This work was supported by the Max Planck Society, the National Science Foundation (#0800793 and #0926190); the National Institutes of Health; by IFCPAR; by BMBF; by the Kavli Institute for Theoretical Physics and by the European Community's Seventh Framework Programme FP7/2007–2013 through HEALTH-F2-2009-241526 (EUTrigTreat).