First patient in China implanted with rechargeable neurostimulator for chronic pain
17 December 2009
A 62-year-old man from Shenzhen, Guangdong province has become the first patient in China to be implanted with St Jude Medical's Eon neurostimulator, a rechargeable device used to help manage chronic pain. Despite prior back surgeries, the patient suffered from chronic back pain for more than a decade.
The Eon neurostimulator, which is the first rechargeable spinal cord stimulator to be approved for use in China, was recently approved by the Chinese State Food and Drug Administration (SFDA) for the management of chronic low back pain and pain from back surgeries that have failed.
“Chronic pain is a serious health issue in China,” said Professor Zhang De Ren, M.D., an interventional pain physician at the Shenzhen Nanshan Hospital of Shenzhen, Guangdong province who performed the procedure. “We are excited to be able to provide an advanced therapy such as neurostimulation in order to improve patient outcomes.”
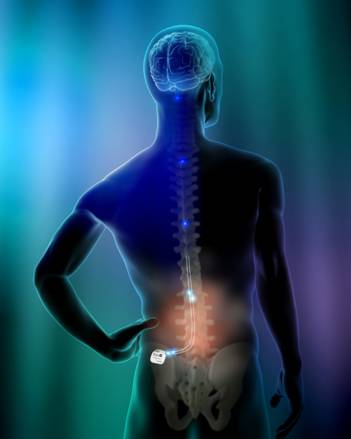 The
Eon neurostimulator is designed to provide spinal cord stimulation
therapy. Similar to a cardiac pacemaker, this “pacemaker for pain”
delivers mild electrical pulses to a lead or leads that are placed in
the epidural space near the spine to interrupt or mask the transmission
of pain signals to the brain (see image on right). Once activated, the
system’s programs are adjusted and fine-tuned to best manage the
patient’s pain.
The
Eon neurostimulator is designed to provide spinal cord stimulation
therapy. Similar to a cardiac pacemaker, this “pacemaker for pain”
delivers mild electrical pulses to a lead or leads that are placed in
the epidural space near the spine to interrupt or mask the transmission
of pain signals to the brain (see image on right). Once activated, the
system’s programs are adjusted and fine-tuned to best manage the
patient’s pain.
“Implanting the first patient in China with a rechargeable neurostimulator represents an important step toward broadening the availability of this therapy,” said Chris Chavez, president of the St. Jude Medical Neuromodulation Division. “We are proud to provide physicians access to technology that can deliver sustainable relief for chronic pain sufferers who may have exhausted other therapy options.”
Neurostimulation patients can adjust the therapy by using a handheld device (similar to a remote control) that allows them to select from pre-set programs that are individually customized. Patients with a rechargeable Eon neurostimulator periodically recharge their devices, potentially resulting in fewer battery replacement surgeries.
In addition to the Eon rechargeable neurostimulator, the SFDA also approved the non-rechargeable Genesis neurostimulator. Non-rechargeable (also known as conventional) neurostimulators provide a convenient option for chronic pain patients who prefer or require the simplicity of a non-rechargeable medical device.
Chronic pain is a largely under-treated and misunderstood disease that affects millions of patients worldwide. The World Health Organization, in conjunction with the International Association for the Study of Pain (IASP), reports that as many as one in five people suffers from moderate to severe chronic pain.