|
|
|
| General care | |
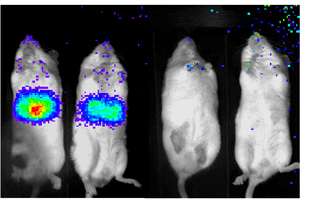
Glowing mice illuminate diabetes26 September 2007 Mice given a customised firefly gene that causes their livers to glow in the presence of key chemicals formed during glucose metabolism could help the development of new diabetes drugs. Researchers at the Salk Institute for Biological Studies, California, report in a study published in Nature that a protein called TORC2 serves as a key biochemical control point linking feeding, insulin, and elevated blood sugar production in the liver. With the help of the luciferase gene from the firefly they made the livers of mice actively producing glucose light up. The findings highlight TORC2 and an enzyme called SIK2 as potential drug targets for treating type II diabetes.
Diabetes is an incurable disease that can lead to blindness, kidney failure, heart disease, and other serious debilitations. According to the International Diabetes Federation, it affects an estimated 246 million people worldwide and every year 3.8 million people die from it. However, up to 80% of type 2 diabetes is preventable by adopting a healthy diet and increasing physical activity. According to the US Centers for Disease Control and Prevention, one in three Americans born in 2000 will develop diabetes. The problem starts during the process by which blood sugar is produced in the liver — gluconeogenesis, explains Marc Montminy, PhD, a professor in the Clayton Foundation Laboratories for Peptide Biology, who led the study. During fasting, gluconeogenesis maintains blood sugar levels by increasing glucose production. After a meal, the hormone insulin normally turns down gluconeogenesis, ensuring that blood sugar levels don’t rise too high. “But in people with insulin resistance, blood sugar levels are elevated because gluconeogenesis continues when it shouldn’t, increasing the risk of developing type II diabetes,” Montminy says. Twenty years ago, Montminy discovered a metabolic switch, a protein called CREB that responds to various physiological signals by turning on and off different gene networks in the body. During periods of fasting when blood sugar stocks run low, for example, CREB turns on gluceoneogenesis in the liver. Recently, Montminy’s team identified a second essential component of the CREB switch, a protein called TORC2, that binds to CREB and enables the switch to work. While the researchers had established that TORC2 was essential to sugar production during fasting, they were keenly interested in understanding the protein’s role during feeding. That knowledge might clue them in to the causes of insulin resistance. To learn how feeding and insulin affect TORC2, the researchers, led by post-doctoral fellows Renaud Dentin and Yi Liu, inserted into laboratory mice the luciferase gene, which produces the glow of firefly tails. They altered the gene so that it could only be turned on in the liver by the CREB/TORC2 switch. When the gene is turned on, the luciferase enzyme causes the liver to light up. Using a sensitive camera, the light — a direct measure of CREB/TORC2 activity — can be detected and measured from outside of the live mice. Using biochemical and genetic techniques to change the levels of various molecules in the pathway, including insulin and TORC2, the researchers measured the effect of these changes on the amount of light emitted from the liver. The experiments revealed that the rise in insulin during feeding turned off the CREB/TORC2 switch. Insulin first activated a liver enzyme called SIK2, which in turn inactivated TORC2 by chemically tagging it with a phosphate group. The extra phosphate group caused TORC2 to leave the cell nucleus where it needed to be to turn on genes. Once the protein left the nucleus, the researches discovered, it was destroyed by the cell’s protein degradation machinery or proteasome. “You need TORC2 to be downregulated by phosphorylation during feeding. If you don’t do that, then the whole gluconeogenic program stays on, and glucose levels go up,” Montminy says. Understanding these key roles of TORC2 and SIK2 points to their potential as drug targets for stemming the rising tide of fasting blood sugar. But the glowing mice might one day do more than illuminate the relationship between feeding, fasting, and the CREB/TORC2 switch. “Because the luciferase mice provide a direct look at glucose metabolism in the liver, this imaging approach may be useful in evaluating potential drugs for the treatment of type II diabetes,” says Montminy.
|