| Diagnostic imaging | ||
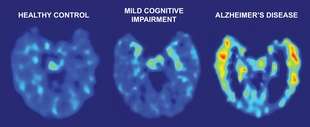
Brain imaging identifies early Alzheimer’s3 January 2007 A new chemical marker developed by researchers at the University of California, Los Angeles (UCLA), enables PET scans to highlight abnormal brain proteins in patients years before Alzheimer’s can be diagnosed with current methods. The study is published in the Dec. 21 2006 New England Journal of Medicine. Scientists are in the early stages of identifying biomarkers in the blood and spinal fluid to help with Alzheimer’s diagnosis, but this new study is the first to report a real time “window into the brain” that identifies both of the major abnormal deposits of the disease in living people who may not develop Alzheimer’s for years to come. The researchers used positron emission tomography (PET) imaging with a small molecule invented at UCLA that binds to the abnormal proteins — amyloid plaques and tangles — that may cause the disease. Previously only an autopsy could determine these deposits and confirm a definitive diagnosis.
The new method was able to track disease progression over a two-year period and was more effective in differentiating patients with Alzheimer’s disease and mild cognitive impairment from normal study subjects when compared to conventional imaging techniques. Researchers are working with Siemens Medical to begin a clinical trial using this new molecular marker in order to obtain Food and Drug Administration (FDA) approval so that it will be available in the future for use by physicians with their patients. “The study suggests that we may now have a new diagnostic tool for detecting pre-Alzheimer’s conditions to help us identify those at risk, perhaps years before symptoms become obvious,” said Dr. Gary Small, Parlow-Solomon Professor on Aging, lead study author and a professor with the Semel Institute for Neuroscience and Human Behavior at UCLA. “This imaging technology may also allow us to test novel drug therapies and manage disease progression over time, possibly protecting the brain before damage occurs.” The study included 83 volunteers aged 49 to 84. Based on cognitive testing, 25 patients had Alzheimer’s disease, 28 had mild cognitive impairment and 30 were normal controls. Researchers performed PET brain scans after intravenously injecting the
volunteers with the new chemical marker called FDDNP, the molecule that
binds to the plaque and tangle deposits found in Alzheimer’s disease. Scientists found distinct differences among people with normal brain aging, patients with Alzheimer’s disease and people with mild cognitive impairment. The PET imaging showed that the more advanced the disease the higher the FDDNP concentration in areas where the abnormal protein deposits typically accumulate — in the temporal, parietal and frontal brain regions. Patients with Alzheimer’s disease showed the most FDDNP binding, indicating a higher level of plaques and tangles than other subjects. “We could see the definitive patterns starting early in patients with mild cognitive impairment and advancing in those with Alzheimer’s disease,” said Dr. Jorge Barrio, study author and professor of medical and molecular pharmacology, David Geffen School of Medicine at UCLA. All subjects also received a PET brain scan using a more conventional chemical marker called FDG, which measures the metabolic function of cells and has previously been used in aiding diagnosis for Alzheimer’s disease. However, FDG cannot identify the abnormal brain protein deposits that may cause the disease. In addition, 72 subjects received magnetic resonance imaging (MRI) scans which show brain structure and size. Scientists found that FDDNP-PET differentiated between study subject groups better than did the FDG-PET combination or MRI. “FDDNP yielded excellent diagnostic accuracy and precisely predicted disease progression and brain pathology accumulation,” said Barrio. “FDDNP-PET also delivers the promise of new drug monitoring in human subjects for a more rapid introduction of therapeutic candidates to control or slow progression of the disease.” Researchers performed follow-up scans two years later on 12 research subjects using FDDNP-PET. Patients who grew worse – declining from normal cognitive function to mild cognitive impairment or from mild cognitive impairment to Alzheimer’s disease – showed an increase of FDDNP binding between five and 11 percent compared with their previous brain scans, suggesting an increase in plaques and tangles. A brain autopsy completed on a follow-up Alzheimer’s patient who died 14 months later showed high plaque and tangle concentrations in areas that had previously demonstrated high FDDNP binding values on the PET scan. “This is the first time this pattern of plaque and tangle accumulation has been tracked in living humans over time in a longitudinal study,” said Small.
|