| Business | |
Oxford BioSignals receives approval for BioSign patient monitor in US and Europe17 May 2006 Oxford BioSignals has received a CE Mark for its BioSign patient monitor, allowing the product to be sold in the European Economic Area and, following its recent US Patent approval, the US Food and Drug Administration has approved its 510(k) application to market BioSign in the United States.
Matthew Walls, Chief Executive Officer of Oxford BioSignals said, “After many years in development we are pleased to announce that, in receiving the CE Mark and gaining FDA approval for BioSign, we are now able to sell this significant new technology in two of the world’s most advanced healthcare markets.” BioSign is intended to help healthcare professionals and Outreach Teams to identify patients in crisis and intervene earlier to correct the problem. Triggered by BioSign, earlier intervention related to a downturn in patient condition is expected to lead to reduced ‘emergency code-calls’ and lower mortality within the hospital. An Outreach or Medical Emergency Team (MET) is summoned when a patient’s condition deteriorates. In the case of a potential cardiac arrest, the Outreach Team can intervene to save lives among patients who may otherwise experience cardiac arrest, by rapidly changing care to prevent the arrest or by facilitating the transfer to an intensive care unit (ICU). Matthew Walls added, “Traditional patient monitoring focuses on collecting and displaying individual vital signs. BioSign focuses on advanced interpretation of vital sign data to generate new clinical information and insight. The patented technology uses data fusion to assess patterns of multiple vital signs to identify patient changes that may not be derived by looking at each vital sign individually.” The first BioSign clinical trial, lasting two and half years, was recently successfully completed at John Radcliffe Hospital in Oxford UK.
|
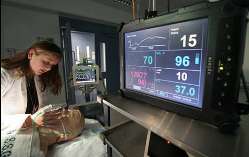 BioSign
is an accessory device to multi-parameter patient monitors that analyses
five vital signs (heart rate, oxygen saturation, blood pressure, temperature
and respiration rate) and assigns a Patient Status Index which continuously
measures how abnormal a patient’s vital signs are with respect to normal
patient conditions.
BioSign
is an accessory device to multi-parameter patient monitors that analyses
five vital signs (heart rate, oxygen saturation, blood pressure, temperature
and respiration rate) and assigns a Patient Status Index which continuously
measures how abnormal a patient’s vital signs are with respect to normal
patient conditions.