Digital pathology under the microscope
10 March 2010
Radiology and pathology are cornerstones of cancer diagnosis, the former allowing tumors to be accurately located and biopsied and the latter providing confirmation of their malignancy.
Radiology has already ‘gone digital’ and without that process having taken place most of the computer-aided imaging modalities that radiologists currently rely on would not be possible.
Today, radiologists are just as comfortable looking at radiology images on computer screens as they are examining a conventional film X-ray on a light box. The same cannot, however, be said for the world of pathology.
In terms of current practice, pathologists still mount tissue slices on glass slides, treat them with appropriate stains and examine them through a microscope. Despite advances in staining techniques, it’s a process that has changed little over the last twenty years. Interpreting what they see is a time-consuming process and requires a great deal of skill and experience. Few tools exist to accurately quantify features of the images that they see through their microscopes.
Increased demand
There is an ever-increasing demand for pathology-based diagnosis, particularly in oncology. The demographic shift to older age-groups means there will be more people suffering from disease, much of it cancer. In addition, today’s high levels of obesity will expose a greater proportion of the population to the risk of cancer at an earlier age. On top of these factors, the number of therapy options becoming available will demand better patient stratification and hence more pathology tests per patient.
However, this increased demand is not currently being matched by the supply of skilled pathologists, who remain in short supply in most parts of the world. There is, therefore, a real need for technology that both improves the efficiency of pathology labs and helps pathologists with their diagnoses.
Going digital
It might be assumed that digitization of pathology images, similar to that which has been embraced in radiology, is the obvious way to go. However, according to Professor Ian Ellis, Professor of Cancer Pathology in the School of Molecular Medical Sciences at Nottingham University (Nottingham, UK), it’s not that straightforward.
“The real driver behind digital radiology was the elimination of wet-film processing, so that you could go instantly from scan to image. Image storage and retrieval via PACS then became add-on benefits,” he says.
“With histopathology, however, you always start with biopsy tissue that needs to be prepared, sectioned and mounted on a glass slide before it can be viewed, and in the majority of cases we still store the original sample in its paraffin block so that it can, if necessary, be re-sectioned in the future.”
That’s not to say he doesn’t already use digital pathology images in his day-to-day work on breast cancer diagnosis. “Quite often when I’m teleconferencing to discuss a case, I’ll put a slide into the digital microscope and share the images,” he says, “but the file sizes for high-resolution images are typically so large that I’m very selective in what I choose to communicate.”
So if the arguments for digital pathology are not quite so straightforward as they are for digital radiology, where will digital pathology reap benefits?
“I still believe that the best machine for interpreting pathology images, for differentiating between significant features and artifacts, is a skilled human being,” says Professor Ellis. “However, where digitization of the images could provide real benefits is in helping to quantify what we see under the microscope, for example, putting numbers to the depth and area of staining or helping to dimension specific image features.”
This highlights a very important feature of future digital pathology systems. If they are to be used for these routine ‘per-slide’ tasks, they need to fit seamlessly into existing pathology workflows and be very fast in terms of their image capture, storage, retrieval and image analysis capabilities.
“Few pathology labs will be prepared to switch overnight to a completely new system,” says Professor Ellis, “so digital pathology needs to be introduced alongside existing processes and procedures in a way that enhances what pathologists can do, yet without slowing them down.”
Rising to the challenge
Leveraging its existing expertise in radiology imaging and PACS (picture archiving and communications systems), Philips is developing an integrated digital pathology system that addresses these workflow requirements.
To meet the need for high-speed image capture, the front-end of the system comprises a highly automated slide scanner that has been designed to scan standard pathology slides at a speed of one slide every 50 seconds (total scanning and handling time). Featuring 40x optical magnification and a high-resolution image sensor, the scanner matches the image quality seen through conventional pathology microscopes.

A Philips prototype pathology slide scanner
Tissue slices are never perfectly flat, which means that pathologists typically have to make fine-focus adjustments as they examine a slide under the microscope. To overcome this surface irregularity problem in the scanner, the Philips scanner employs dynamic autofocus techniques, originally developed by the company for Blu-ray Disc players, to accurately follow the tissue section’s surface contours. As a result, the captured images maintain accurate focus across the entire image plane, which is an essential requirement for subsequent viewing of the stored images on a monitor.
Even though a pathology slide is relatively small (typically 1.5 cm x 1.5 cm), capturing the image in sufficient detail requires the use of an image sensor with hundreds of millions of pixels and generates large image files — typically tens of Gigabytes of raw data.
The Philips slide scanner is therefore coupled to a high-speed high-capacity image storage and viewing system that has been specifically designed to efficiently store, retrieve and view the digitized pathology images. To assist pathologists in the analysis and interpretation of these images, the system also includes image analysis software for feature recognition and quantification. The software will also act as a platform for developing additional functionality that was not possible before, such as focusing at different depths in tissue samples to build up 3D pictures of their internal structure.
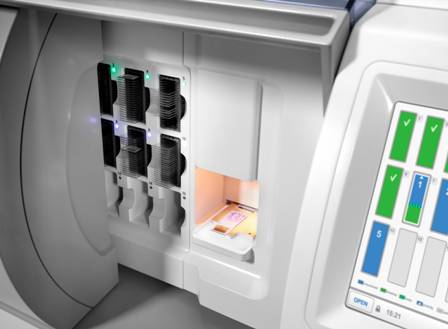
A glass slide in a prototype slide scanner
Immediate uses
Although Professor Ellis believes that digital pathology systems will need to be phased in alongside conventional pathology methods and procedures so that pathologists can become familiar with them and recognize their benefits, he believes there are immediate applications where digital pathology could prove extremely useful.
“As part of our research into the immunophenotypic characterization of protein expression in breast cancers we are already using tissue arrays — hundreds of tissue samples mounted on a single slide — to directly compare biopsy material from large patient cohorts,” he says. “When it comes to these highly repetitive quantitative analysis tasks, experience has shown that computers make fewer mistakes than human beings.”
He also believes that digital pathology could be of immediate value for training purposes, where large numbers of students need to view the same slides.
New ways of working
The other value of digital pathology will be to enable new ways of working that improve the efficiency of pathology services. For example, it would mean that seeking the expert opinion of an external specialist no longer involves physically sending slides to them.
It could also enable pathology departments to restructure their organizations — for example, eliminating the need for lab technicians to be located alongside pathologists in the same facility.
The ability to rapidly communicate images between facilities could even allow the setting up of distributed pathology services based around virtual networks. Philips is already working with leading pathology departments to evaluate the benefits of such systems in real-world clinical environments.
However, in order to fully exploit the benefits of digital pathology, there is still much to be done. For example, no industry standard comparable to DICOM (digital imaging and communications in medicine — a standard for handling, storing, printing and transmitting information in medical imaging), currently exists for digital pathology images. Due to the file sizes involved, it will also be necessary to develop artifact-free image compression techniques and/or faster IT infrastructures.
Philips therefore welcomes partnerships with organizations that can help with such developments. It is also actively seeking collaborations with companies that specialize in histological stains, staining techniques and other histopathology procedures in order to develop the system further.
Further information
Information on research being conducted by Professor Ian Ellis’s
group at the University of Nottingham (Nottingham, UK) can be found
at:
www.nottingham.ac.uk/mol/lookup/lookup_role.php?id=NjAxMzAx&page_var=personal
More information about Philips Digital Pathology Systems can be found on www.philips.com/digitalpathology.